1. Extraction and Separation of H3 Variant Histones (See Table 3 for protein sequences.)
- Extraction from whole cells by sonication in 40% guanidine.HCl, batch-wise chromatography on BioRex-70 resin in 5% and 40% guanidine.HCl and dialysis into dilute acetic acid [1-2]
- H3 purification by Reversed-Phase hplc: Fig. 1 [2-3]
- H3 variant separation by AUT gel analysis: determination of optimum Triton X-100 concentration by AU-AUT gradient gel analysis: Fig. 2 [4]
|
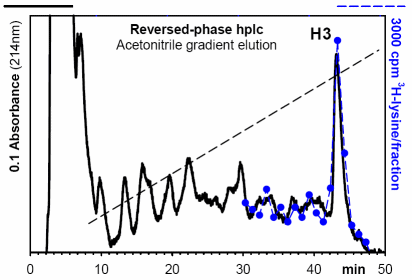 Figure 1. Purification of histone H3 by Reversed-Phase hplc from a crude guanidine/BioRex-70 extract from 5 x 109 cells of Ustilago maydis, labeled in vivo for 30 min with 50 uCi [3H]-lysine.
Figure 1. Purification of histone H3 by Reversed-Phase hplc from a crude guanidine/BioRex-70 extract from 5 x 109 cells of Ustilago maydis, labeled in vivo for 30 min with 50 uCi [3H]-lysine.
|
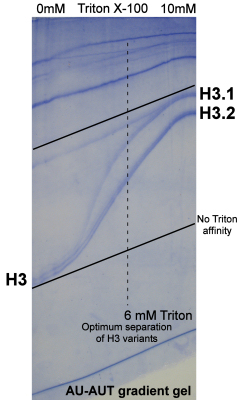
Figure 2. Separation of hplc-purified Ustilago histone H3 sequence variants by differential affinity for Triton X-100 in acid-urea gel electrophoresis.
The co-electrophoresis of the two sets of bands in the absence of Triton and separation by Triton X-100 defines these proteins as core histone variants. Standard variant nomenclature gives a .1 extension to the lowest mobility form, .2 to the form with the next highest affinity, etcetera.
|
2. Study of Ustilago Histone H3 Variant Features
- During growth, H3.1 contains about 33% of all H3 protein (Fig 3, lanes A, C) with, on average, 1.2 acetylated lysines per protein, typically 10-20% higher than the level in H3.2.
- Synthesis of histone H3 with [35S] methionine only labels the H3.2 form (lane B), identifying H3.2 as the product of gene U2 and the mature, methionine-free H3.1 as the product of gene U1 (see Table 3).
- Tritated lysine labels both H3 variants (lane D) with 60% of the label incorporated into new H3.1 histone, when H3.1 steady state levels are much lower at about 30%. As with methionine labeling, newly synthesized H3 protein appears highly acetylated (on average 2.7 and 2.4 acetylated lysines per new H3.1 or H3.2, respectively) with undetectable (H3.2) or low levels (5% in H3.1) of unacetylated forms.
- Chase of lysine-labeled cells for 24 hours into stationary phase shows a number of changes:
- The relative abundance of H3.1 increases from 33% to 49% of total H3. This is consistent with continued synthesis of H3.1 protein and cessation of H3.2 synthesis when cell proliferation stops.
A more detailed analysis of this change is shown below.
Steady-state acetylation levels drop 10 and 25% in H3.1 and H3.2, respectively, consistent with decreased gene expression under stationary phase conditions.
- The label pattern (lane F) has changed to the steady-state pattern (lane E).
- Turnover of histone H3 protein is suggested by the loss of radioactivity (compare lanes D and F).
- When grown into stationary phase, H3.1 levels increased to 49% (lane E) with a 10 to 25% decrease in acetylation levels of H3.1 and H3.2, respectively.
- Under conditions of fast growth, the relative amount of H3.1 will decrease to levels as low as 20-22% (Fig. 4, lanes A, C) with increased acetylation levels of 1.3-1.6 acetylated lysines per protein. This level is typically 20-30% higher than measured for histone H3.2.
- Short pulse labeling with tritiated acetate under conditions of complete inhibition of protein translation by cycloheximide excludively identified post-synthetically the mono- through hexa-acetylated forms of both H3 variants with 36% of the label in H3.1 (lane B). The specific radioactivity of H3.1 was 2.1 times higher than that of H3.2 and, on average, H3.1 contained 3.4 tritiated acetylated lysines per H3.1 vs 2.4 per H3.2. All these characteristics support the notion that H3.1 is preferentially localized in transcriptionally active chromatin regions.
- Longer pulse labeling with acetate, e.g. for 20 min (lane D), results in lower label incorporation, consistent with the high rate of turnover of the acetyl moieties, with half-lifes estimated to be in the order of just a few minutes, even though no formal pulse-chase experiment is performed.
This behavior was also observed for acetate labeling in Chlamydomonas [5]. Studies in other organisms has shown the fastest turnover rates in actively transcribed chromatin loci [6].
The decrease of H3.1 acetate labeling from 5 to 20 min from 36% to 17% of total labeled H3 (when the steady-state level of H3.1 is about 20-22%) shows that acetate turnover of H3.1 is faster than of H3.2, consistent with its preferential localization in transcriptionally active chromatin.
|
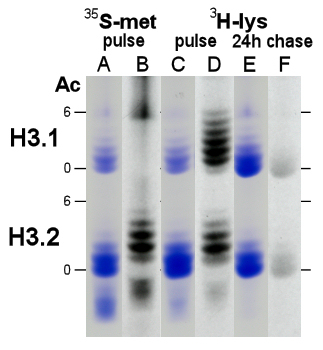
Figure 3. AUT gel analysis of Ustilago histone H3, stained with Coomassie Blue (lanes A, C, E) and fluorographed for 33 days (lanes B, D, F) from cells labeled for 20 min with 25 uCi [35S]-methionine (lanes A-B) or labeled for 30 min with 50 uCi [3H]-lysine (lanes C-D) and chased for 24 hours into stationary phase culture (lanes E-F). Gel mobility of non- (0) and hexa-acetylated (6) H3 variant forms is marked.
|
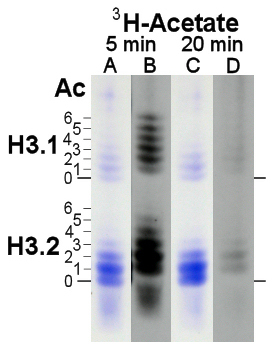
Figure 4. AUT gel analysis of Ustilago histone H3, stained with Coomassie Blue (lanes A, C) and fluorographed for 18 days (lanes B, D) from cells labeled for 5 min (lanes A-B) or 20 min (lanes D-E) with 1 mCi [3H]-acetate under conditions of inhibition of protein translation (10 ug/ml cycloheximide). Gel mobility of non- (0) through hexa-acetylated (6) H3 variant forms is marked.
|
3. Study of Cell Cycle-modulated Expression of Histone H3 Variants
- Progression of Ustilago cultures from logarithmic proliferation and growth to non-proliferative steady-state results in an increase of the relative amount of histone H3.1 from about 30% to about 50% in 12 hours (Fig. 5A). The same change was observed during the chase of lysine-labeled cells (Fig. 3, lane C to lane E).
Steady-state acetylation levels gradually dropped with acetylation of H3.1 typically 10% higher than in H3.2 (Fig. 5B).
- Cell cycle synchronization of logarithmically growing Ustilago cultures (doubling time: 2 hours) was achieved by imposing and releasing a DNA replication block by hydroxyurea [7]. Tritiated lysine labeling was completely inhibited in the presence of hydroxyurea, peaked shortly after release from the block during replication and this was followed by a sharp rise in cell density as cells progressed through mitosis.
As expected after 2 hours, the length of one cell cycle, a second but lower peak of lysine incorporation was observed (Fig 6). This demonstrates that replication-dependent expression of histone H3 exists in Ustilago.
- The high 'background' of lysine incorporation into histone H3 outside of S phase (measured after H3 purification by hplc but before separation of H3 variants by AUT gel electrophoresis) suggests also the presence of significant constitutive expression of histone H3.
Once exposures have been completed, fluorography of purified H3 variants, separated by AUT gel electrophoresis, will show whether the S phase peak and the high non-S lysine labeling are specific for just the H3.2 and the H3.1 variant, respectively, or whether both characteristics co-exist in one or in both forms.
|
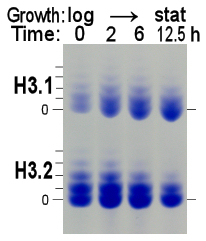 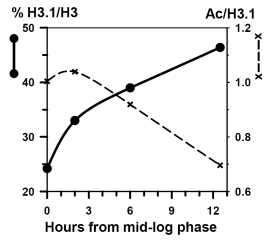
Figure 5. Left panel: AUT gel analysis of histone H3, purified from log-phase cultures (time = 0 h; cell density ~0.4 x 107 cells/ml) which doubled every 2 hours, and after 2, 6 and 12.5 hours continued culturing to stationary phase (cell density ~2.5 x 107 cells/ml). Right panel: Increase in the relative abundance of histone H3.1 concomitant with a drop in histone H3.1 acetylation (shown) and a similar decrease in H3.2 acetylation (not shown) as cells reach non-proliferative, stationary phase conditions.
|
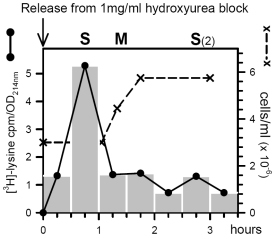
Figure 6. Histone H3 labeling with [3H]-lysine of Ustilago cells, arrested in mid-log phase by a replication block of 1 mg/ml hydroxyurea and released by medium replacement at time zero. Specific activity was determined in hplc fractions as cpm per absorbance unit at 214nm. Culture density was monitored by hemocytometer cell counting.
|
|
References
- Mende LM, Waterborg JH, Muller RD, Matthews HR. "Isolation, identification and characterization of histones from plasmodia of the true slime mold Physarum polycephalum using extraction with guanidine hydrochloride." Biochemistry 22, 38-51, 1983.
- Waterborg JH. "Histone synthesis and turnover in alfalfa. Fast loss of highly acetylated replacement histone H3.2" J. Biol. Chem. 268, 4912-4917, 1993.
- Waterborg JH. "Steady-state levels of histone acetylation in Saccharomyces cerevisiae." J. Biol. Chem. 275, 13007-13011, 2000.
- Waterborg JH. "Acid-urea-Triton polyacrylamide gel electrophoresis." in "The protein protocols handbook, 2nd edition" (Walker JM, ed) Humana Press NJ, pp. 113-123, 2002.
- Waterborg JH. "Dynamics of histone acetylation in Chlamydomonas reinhardtii." J. Biol. Chem. 273, 27602-27609, 1998.
- Waterborg JH. "Dynamics of histone acetylation in vivo. A function for acetylation turnover?" Biochem. Cell Biol. 80, 363-378, 2002.
- Garcia-Muse T, Steinberg G, Perez-Martin J. "Pheromone-induced G2 arrest in the phytopathogenic fungus Ustilago maydis." Eukaryotic Cell 2, 494-500, 2003.
|
|
 Figure 1. Purification of histone H3 by Reversed-Phase hplc from a crude guanidine/BioRex-70 extract from 5 x 109 cells of Ustilago maydis, labeled in vivo for 30 min with 50 uCi [3H]-lysine.
Figure 1. Purification of histone H3 by Reversed-Phase hplc from a crude guanidine/BioRex-70 extract from 5 x 109 cells of Ustilago maydis, labeled in vivo for 30 min with 50 uCi [3H]-lysine.





